Franz X. Kärtner | Deutsches Elektronen-Synchrotron DESY | Hamburg, Germany
“From Precision Timing of X-FELS to Nonlinear Microscopy”
Scientific Talks, Session IV | Tuessday, September 13 | 9:00 – 9:30

Summary: Over the last decade, X-ray Free-Electron (X-FEL) Lasers have made big strides towards structure determination and studying time resolved dynamics of biological macromolecules by developing serial femtosecond X-ray crystallography. The facilities and the science critically depends on the latest developments in femtosecond laser technology. In this presentation, we discuss two areas where femtosecond lasers have enabled major advances. These are large-scale timing distribution and synchronization of the X-FEL facilities and scoring of protein micro- and nano-crystals by nonlinear microcopy. The most advanced X-FEL facilities the European X-FEL, LCLS and soon to be LCLS-II and SHINE measure up to 3.5 km in length and are in need for precision timing and synchronization systems enabling permanent sub-10 fs synchronization of optical and RF-sources to exploit the full science potential of these infrastructures. With the upcoming attosecond pulses from X-FELs there will be soon need for sub-femtosecond performance in the optical domain. We will describe the principles of a pulsed optical timing system developed over two decades that enables this performance and is now implemented or planned to be implemented in most X-FEL facilities [1,2].
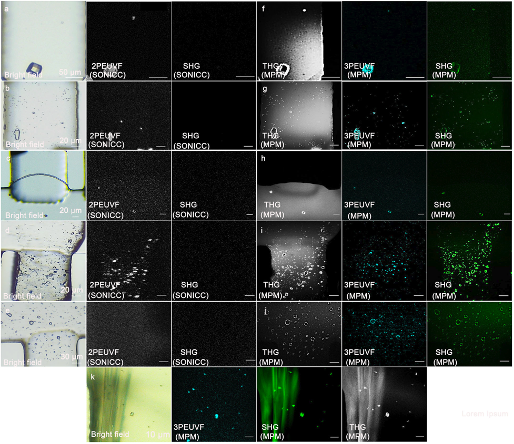
Fig. 1 Images of different in vitro protein crystals selected for imaging experiments. The method of detection and imaging is indicated in the lower left of each image. a–e SONICC imaging of a large lysozyme crystal, micro-sized lysozyme crystals, micro-sized proteinase K crystals, micro-sized thaumatin crystals, and micro-sized thermolysin crystals. f–j Multimodal multiphoton imaging methods for lysozyme crystals, micro-sized lysozyme crystals, micro-sized proteinase K crystals, micro-sized thaumatin crystals, and micro-sized thermolysin crystals. k Multimodal multiphoton imaging methods for micro-sized thaumatin crystals and sodium-tartrate crystals.
After being able to trigger chemical and biological reactions of molecules with light and to image the corresponding molecules in motion with femtosecond X-ray flashes to study the dynamics underlying its function, there is an increasing demand for effective and in time methods to identify, detect and score protein micro- and nano-crystal suspensions for serial diffraction data collection at X-FELs as well as high-intensity microfocus synchrotron radiation sources. Jointly with the group of Ch. Betzel we worked closely together to demonstrate a compact multimodal, multiphoton microscope [3], driven by a femtosecond fiber laser [4], enabling excitation wavelengths at 775 nm for SHG/UV imaging, 1300 nm for THG imaging, which for the first time simultaneously records second-harmonic generation, third-harmonic generation and ultraviolet fluorescence to identify and detect protein crystals with high sensitivity, see Fig. 1. The instrument is most valuable, unique and important for scoring and sample optimization in biomolecular crystallography to detect small crystals grown in vitro or in vivo and capable to distinguish between salt and protein crystals. Also applications to imaging of human skin and brain tissue will be discussed.
References
[1] J. Kim, J. A. Cox, J. J. Chen, and F. X. Kärtner, “Drift-free femtosecond timing synchronization of remote optical and microwave sources,” Nature Photonics 2, 733-736 (2008), doi:10.1038/nphoton.2008.225
[2] M. Xin, K. Shafak, M. Y. Peng, A. Kalaydzhyan, W. Wang, O. D. Mücke, F. X. Kärtner, “Attosecond Precision Multi-km Laser-Microwave Network,” Light: Science and Applications (2017) 6, e16187; doi:10.1038/lsa.2016.187
[3] Q. -D. Cheng, H. –Y. Chung, R. Schubert, S. -H. Chia, F. X. Kärtner, M. Perbandt, G. Q. Chang, and C. Betzel, “Multiphoton microscopy for nanocrystal detection and scoring driven by a fiber-based ultrafast source,” Communication Biology 3:569 (2020) | https://doi.org/10.1038/s42003-020-01275-8.
[4] H. –Y. Chung, W. Liu, Q. Cao, R. Greinert, F. X. Kärtner, and G. Q. Chang, “Novel fiber-based ultrafast source tunable between 1.15 μm and 1.35 μm for harmonic generation microscopy in human skin,” IEEE J. Sel. Topics in Quantum Elec. 25 (1) 1-8 (2019).